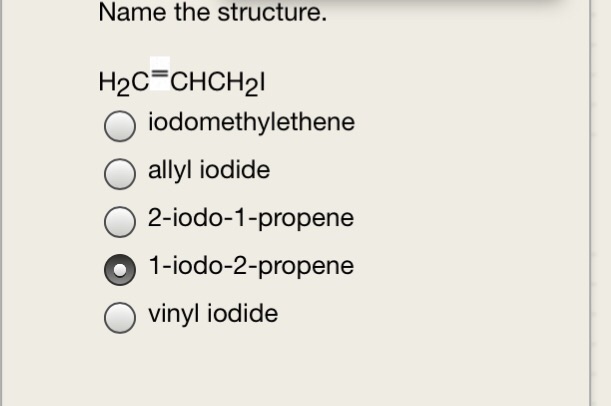
Allyl And Vinyl Structure. Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms. An allyl group is a substituent with the structural formula h2c ch ch2r where r is the rest of the molecule. The key difference between allyl chloride and vinyl chloride is that ally chloride contains its chlorine atom bonded to the carbon atom that is adjacent to the double bond whereas vinyl chloride contains its chlorine atom bonded to one of the two carbon atoms in the double bond.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds. The name is derived from the latin word for garlic allium sativum.
A styrenic crosslinker with two vinyl groups is called divinyl benzene.
An allyl group is a substituent with the structural formula h2c ch ch2r where r is the rest of the molecule. The general molecular formula for allyl is rch 2 ch ch 2 whereas the general molecular formula for vinyl is rch ch 2. Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds. It consists of a methylene bridge attached to a vinyl group.
